Home
HSDiagnomics partners with Charité University Medicine Berlin, TheryCell GmbH, and EPO GmbH in an innovative project to advance TCR-T therapy for solid tumors
Berlin, October 30, 2023 - HSDiagnomics, in collaboration with TheryCell GmbH, EPO GmbH and Charité University Medicine Berlin is excited to launch a cutting-edge project aimed at advancing the identification and preclinical validation of tumor-specific T-cell receptors (TCRs) for TCR-T cell therapy of solid cancers.
Building upon HSDiagnomics' proprietary antigen-agnostic approach successfully validated in Non-Small Cell Lung Cancer (NSCLC), this novel methodology to identify tumor-specific T-cell receptors will now be extended to more solid cancers with high medical need.
The collaborative effort of three Biotech companies and the Dept. of Hematology, Oncology & Cancer Immunology and the Institute of Pathology of the Charité will focus on identifying and validating tumor-specific T-cell receptors for Pancreatic Ductal Adenocarcinoma (PDAC), Colorectal Carcinoma (CRC), and Breast Cancer (BC). This ambitious project aims to advance TCR-T therapies ranging from the selection of therapeutic TCRs to establishing a GMP-compliant workflow for the manufacture of therapeutic doses of personalized as well as off-the-shelf TCR-T cell therapies.
The project is funded for three years by the ProFIT program of the IBB Berlin and the European Union (EFRE, Europäischer Fonds für regionale Entwicklung), underscoring the significance and potential impact of this pioneering initiative.
Pioneers in Quantitative TCRα/β Profiling Since 2012
Founded in 2012, HSDiagnomics is a leader in quantitative T-cell receptor (TCR) profiling. Our proprietary TCRsafe® technology provides detailed insights into the TCRα/β repertoires of blood and tissue samples, including fresh samples and archival paraffin-embedded (FFPE) specimens, down to the single-cell and nucleotide level.
Comprehensive TCR Profiling Services
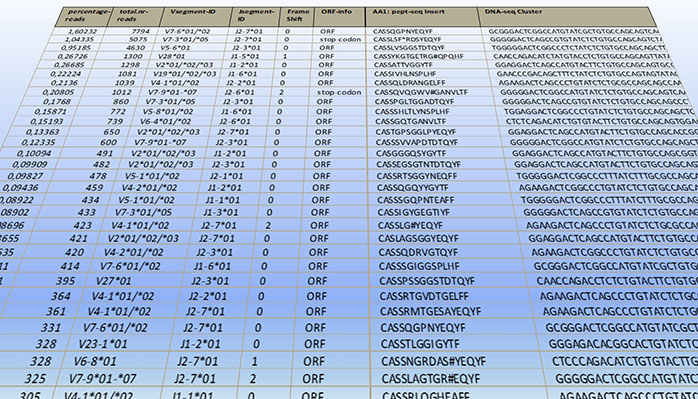
We offer a full-service solution for high-resolution TCR profiling in both human and mouse samples. Our team manages the entire process, delivering quantitative profiles of TCR clonotypes defined by V- and J-segment usage and Complementarity Determining Region 3 (CDR3). These results are presented in user-friendly formats, facilitating further research and supporting applications in diagnostics and personalized medicine.
Your partner for TCR-T therapy development and antigen characterization
HSDiagnomics is your ideal partner for exploring the potential of TCR-T therapies in dedicated tumors. Check our portfolio in tumor-specific TCR-T manufacturing and proposed routes to antigen identification here. The backbone of our highly advanced services for industrial customers is our TCancerR data base of tumor-specific TCRs with significant potential for TCR-T therapies.
With TCanceR HSDiagnomics maintains a unique and high-resolution database of tumor-specific T-cell receptors (tsTCRs), which is sourced from deep comparison of TCR profiles in tumor and adjacent healthy tissue. The methods behind TCanceR are best-in-class and patented. This database is a key resource for detection of common tsTCRs, development of therapeutic TCRs and the identification of unique as well as shared cancer-specific antigens. We focus on solid tumors with high unmet medical need, such as lung, pancreatic, breast and colorectal cancers. A flexible suite of bioinformatics tools can be adapted to customers’ needs and will select most promising tsTCRs for TCR-T and other therapy developments in an unprecedented and efficient way.
Reliable Technology and Expertise for TCR Production and Reactivity Testing
We have developed a streamlined pipeline for synthesis, cloning, transient/stable expression, and functional analyses of disease-specific TCRs across various technologies and diseases. This capability is integral to the production and functional validation of therapeutic TCRs. In partnership with Therycell GmbH we work on a fully synthetic TCR transfection approach (Including the use of non-viral sleeping beauty technology) to develop safe and payable TCR-T therapies.
Collaborations and Impact
Since our founding, HSDiagnomics has been a trusted partner in numerous academic and commercial projects, especially within the fields of immuno-oncology and autoimmune diseases. We maintain active collaborations with clinical partners such as Charité University Medicine and Vivantes Clinics Berlin as well as with applied research institutions like Fraunhofer Institute and Max Delbrück Center. Our focus is on cellular immunotherapies for difficult to treat tumors, i.e. advanced lung, colorectal, pancreatic, and breast cancer.
HS Diagnomics GmbH
Schloßstrasse 110
12163 Berlin
Germany
Tel. +49(30)700 14 53 30
Fax +49(30)546 11 698
Customer Support
Tel. +49(30)700 14 53 31
News
05/2025
Breakthrough Discovery: Shared Tumor-Specific TCRs Open the Door to Scalable, Off-the-Shelf Immunotherapies. A newly published study on bioRxiv (DOI: 10.1101/2025.04.26.650660v1) demonstrates what could be a turning point in cancer immunotherapy.
Read more ...
04/2025
Advancing Cancer Immunotherapy with Novel KRAS-Q61H TCR-T Therapy. HS Diagnomics GmbH, a leader in tumor-specific T-cell receptor (TCR) discovery, is making significant strides in the development of personalized immunotherapies for cancer.
Read more ...
06/2024
Project Update: HS Diagnomics has entered into the international collaborative project ELEVATE with TU Wien, NMI Tübingen, Sciospec, UpNano, and THT Biomaterials
Read more ...
10/2023
HSDiagnomics partners with Charité University Medicine Berlin, TheryCell GmbH, and EPO GmbH in an innovative project to advance TCR-T therapy for solid tumors
Read more ...
06/2020
TCRsafe® analysis of a Runx1 knockout mouse model provides evidence for a role of RUNX1 as a recombinase cofactor for TCRß rearrangements and pathological deletions
Read more ...
08/2019
ZIM-Project TCR-specific Antigen Identification - TAgID
Read more ...
04/2018
Patent granted for the identification of tumor-specific T-cell receptors
Read more ...